The life cycle of cooking oil
Share
What does oil aging mean and why is it critical for your food quality
That the days of a frying oil’s life are numbered once it has been heated up the first time is nothing new and common knowledge. But what is happening to the oil and why and how is the food affected fried in it?
Let us have a closer look at the frying oil, the deterioration process and the frying process itself.
with our cooking oil. All our oils, fats and shortenings, no matter whether from plants or animals are formed by triglycerides – a combination of glyceride molecule and three fatty acids.

When we start heating up our oil and drop the first load of food something happens to our Tri glyceride during the frying process. To be exact, it breaks! Fatty acids connected to the Glycerol separate and stay back as free fatty acids. With the break not only free fatty acids appear but byproducts like ketones or aldehydes increase in numbers. The overall effect these changes and
restructuring of the oil has is an increase of polarity. Therefore, most effective oil
quality testers measure the polarity (TPM) of the oil and not only free fatty acids since the later is not accounting for these byproducts.

So higher polarity is bad for my fried food? Yes and no!
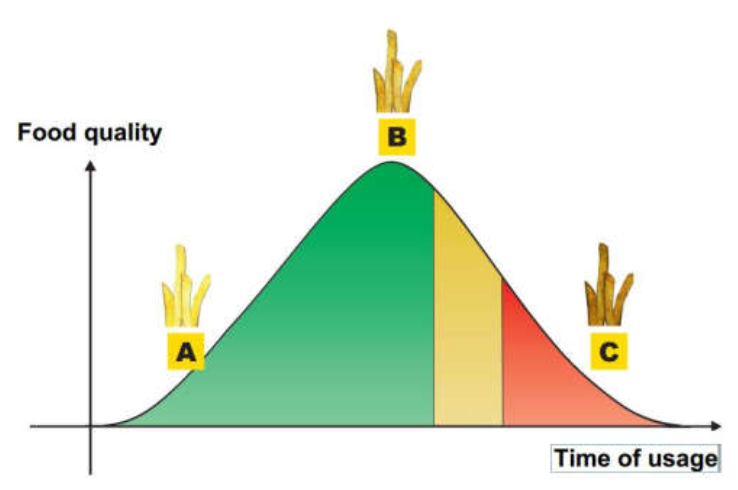
Following graph shows the food quality in comparison to the oil life.

What stands out is that the actual food quality is better in a slightly used oil than a in a fresh oil. The reason for that is the actual process of frying and why our food turns crispy and darker but stays juicy!
Let us imagine you drop a batch of fresh French fries. You hear sizzling and see
steam coming out of the oil. And this steam is at the core of your cooking process. The water in your food is heated by the oil and released from the product into the oil creating steam. The product is slowly drying out but not burning due to the protective shield created by the steam surrounding the product. This shield prevents your fries from being burnt before being cooked thru.
But what has the TPM to do with that? Simply said, it manages how fast the water is getting out of the product and into the oil. With most Triglyceride chains intact, a lower TPM value, it takes the water a lot longer to be absorbed by the oil. The higher TPM value and with that looser chains of molecules make it far easier for the water to evaporate. This leads to the product turning darker and getting crispier faster. Since most of us prefer a darker and crispier French fry over a bland, light and soft one, increased TPM value actually helps us - for some time at least.
As you can see in the graph the optimal outcome is at about halfway through the oil’s life. The ratio of polar material in the oil is just perfect and produces the perfect French fry. Unfortunately, this perfect state is only a short part of the entire life, so staying in that range for as long as possible is what we want. Stretching the oil life can be achieved by using a microfiltration system that ensures the oil being in preferred TPM levels for longer.
To get more information about the life cycle of cooking oil and filtration options and TPM readers, contact us.



